The last weekly tip #9 described how to model a surface with a slab structure under periodic boundary condition and how to create an adsorption structure. The energy in a slab structure can be used to calculate the adsorption energy and surface energy. This weekly tip will cover how to calculate the adsorption energy and surface energy through modeled structures.
1. Calculation of Adsorption Energy
'Adsorption energy' can be defined as the decreasing energy while two materials are combined under the adsorption process in which an atom, ion, or molecule (adsorbate) is attached to the surface of a solid (adsorbent).
This energy is mainly used when calculating the chemical engineering properties, exploring the adsorption mechanism, or determining the energetic heterogeneity of the surface of a solid.[1]
Adsorption energy, Ea, can be calculated as follows, by calculating the difference between the energy of the adsorption model and the sum of the energy which separately calculated for each structure composing the adsorption structure.[2]
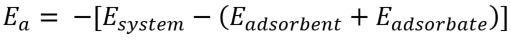
Thus, to obtain the adsorption energy, Ea, we should calculate the energies for the following three models.
- Slab supercell structure (adsorbent)
- Adsorption structure: the slab structure with the adsorbent (adsorption system)
- Adsorbate in the vacuum (adsorbate)
When calculating the energy of the slab structure, it is better to optimize the unit cell first, and make the supercell using the optimized unit cell. As the calculation time of a slab structure relatively long because of the vacuum, the calculation time can be reduced using the optimized structure. For the relaxation of the unit cell, you should use the 'vc-relax' option, including cell relaxation. And it is okay to set the k-point grid as 1 to the axis with a vacuum because the cell length of the axis is sufficiently long.
When calculating an adsorption structure, you should be cautious about the calculating options. Unlike bulk unit cells, its calculation time is significantly longer than that of bulk. Because the adsorbate lowers the symmetry of the model. To decrease the calculation time, you can calculate by dividing it into multiple steps. For example, roughly optimize the structure first, through a calculation of low precision. And next, calculate the structure through a calculation of high precision, again, to obtain a good result.
To find out the energy of adsorbate, you need to place it in enough vacuum size. Because the periodic boundary condition assuming that cell (lattice + basis) is unlimitedly repeated, the interaction between repeated molecules may affect the calculation if the cell size is not enough.
Thus, set the cell size properly and sufficiently so that the distance between periodically repeated molecules is 10 Å or longer. For the calculation of single atoms, read the weekly tip #5 Short but Important Calculation of a Single Atom.
The following shows an example of calculating the adsorption energy.
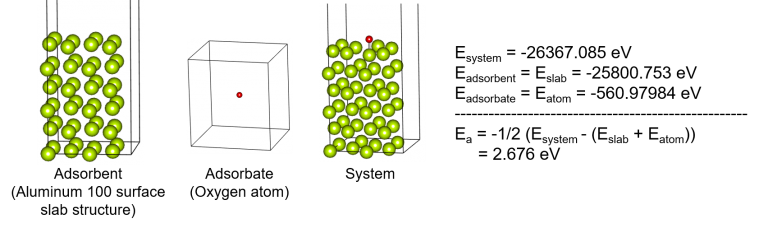
👉 Check the result of calculating the adsorption energy
When an oxygen atom is adsorbed to the Aluminum (001) surface, it is calculated that the adsorption energy is about 2.68 eV, and the adsorption distance is about 1.69 Å. As such, calculating the adsorption energy and comparing the energy and the adsorption distance in a system where atom/molecule is adsorbed on the surface in a specific direction will show which surface with certain characteristics is well adsorbed to an adsorbate.
1. Calculation of Surface Energy
When modeling a slab structure, it is better to use the optimized unit cell to create a slab structure. Then, it can reduce the time for calculating a slab structure. In addition, with the two energies, you can calculate the surface energy. Surface energy can be calculated as follows:[3]
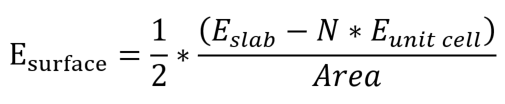
Aluminum has an FCC structure, and its atoms are arranged according to the orientation as follows:
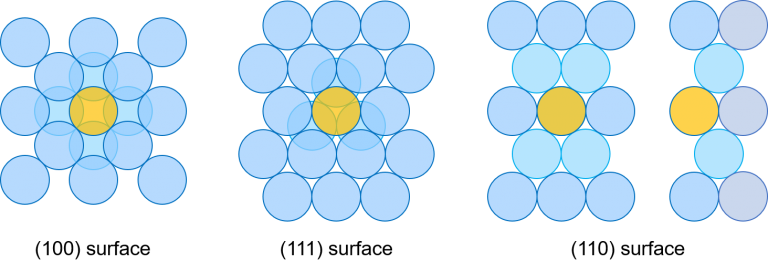
As the number of dangling bonds increases per unit area, the surface energy rises. In bulk, all atoms composing the FCC structure are combined with 12 atoms. However, the atoms on the (001)/(010)/(100) surface are linked with eight atoms. Thus, four dangling bonds are left. The atoms on the (111) surface are combined with nine atoms, and three dangling bonds are left. The atoms on the (110) surface are linked with seven atoms, including the next layer and the layer after the next one, and five dangling bonds are left.[4]
The surface energy depends on the number of layers. If a slab is set to 14 layers, the surface energy of the Aluminum (001) is 0.89 J/m2 and that of the (111) is 0.69 J/m2. The surface energy of the (110) is 0.96 J/m2. These results show that the smaller the number of atoms combined with one atom is, the smaller the surface energy is.
👉 See the surface energy calculation result
Following the last weekly tip that described how to create a slab structure, this weekly tip covers how to use a slab structure to calculate the adsorption energy and surface energy. The two energies are used in various researches. Thus, you can use the data in diverse fields by applying this weekly tip information.
[1] "Glossary". The Brownfields and Land Revitalization Technology Support Center. Retrieved 2009-12-21.; Rouquerol, J., Rouquerol, F., Llewellyn, P., Maurin, G., & Sing, K. S. (2013). Adsorption by powders and porous solids: principles, methodology and applications. Academic press.
[2] Sorescu, D. C., Thompson, D. L., Hurley, M. M., & Chabalowski, C. F. (2002). First-principles calculations of the adsorption, diffusion, and dissociation of a CO molecule on the Fe (100) surface. Physical Review B, 66(3), 035416.
[3] Sun, W., & Ceder, G. (2013). Efficient creation and convergence of surface slabs. Surface Science, 617, 53-59.
[4] Ghetta, V., Gorse, D., Mazière, D., & Pontikis, V. (Eds.). (2008). Materials Issues for Generation IV Systems: Status, Open Questions and Challenges. Springer Science & Business Media.;

0 Comments